



Categories: Peptide Finished product, Peptides and Their Dosages
Free (1) 30 ml Bacteriostatic Water
with qualified orders over $500 USD.
(excludes capsule products, cosmetic peptides, promo codes and shipping)
Tesamorelin, is a growth-hormone-releasing hormone (GHRH) analogue used clinically for the treatment of HIV-associated lipodystrophy (dysfunctional fat deposition). It is also being researched for its ability to improve peripheral nerve health, slow the progression of mild cognitive impairment, and the reduction fat mass.
Product Usage: This PRODUCT IS INTENDED AS A RESEARCH CHEMICAL ONLY. This designation allows the use of research chemicals strictly for in vitro testing and laboratory experimentation only. All product information available on this website is for educational purposes only. Bodily introduction of any kind into humans or animals is strictly forbidden by law. This product should only be handled by licensed, qualified professionals. This product is not a drug, food, or cosmetic and may not be misbranded, misused or mislabled as a drug, food or cosmetic.
Tesamorelin is a growth hormone releasing hormone (GHRH) analogue consisting of standard GHRH to which an additional trans-3-hexanoic acid group has been added. Produced by Theratechnologies of Canada, Tesamorelin became the newest drug to be approved by the FDA for use in HIV-associated lipodystrophy in 2010. The peptide has also been investigated for its ability to improve peripheral nerve regeneration and as a potential intervention for mild cognitive impairment (MCI), the precursor to dementia.
As a GHRH analogue, tesamorelin has all of the same effects as GHRH and GHRH analogues like sermorelin, GRF (1-29), CJC-1295, etc. The addition of trans-3-hexanoic acid to tesamorelin makes it more stable in human plasma and thus increases its half-life. Despite this increase in half-life, tesamorelin, like CJC-1295, preserves the physiological action of GHRH and thus has fewer side effects than similar molecules that obliterate normal pulsatile growth hormone (GH) release.
The primary use for tesamorelin is in the treatment of HIV-associated lipodystrophy, which arises both as a consequence of HIV infection and as a side effect of antiretroviral therapy. In lipodystrophy, fat accumulates excessively both in the abdomen and in other areas of the body. The physiologic mechanism responsible for this is not clearly understood, but it is thought that commonly used protease inhibitors play a large role in the pathogenesis of lipodystrophy[1].
Patients suffering from lipodystrophy initially had diet, exercise, and a handful of ineffective medications to rely on for treatment. If those did not work, surgery was a last-ditch, often ineffective, and frequently complicated solution. In 2010, however, the FDA approved tesamorelin specifically for the treatment of HIV-associated lipodystrophy. The drug has been found to reduce adiposity by nearly 20% in this population [1]. Research suggests that tesamorelin is approximately 4 times more effective in reducing adiposity than all of the other available therapies combined [2].
People with HIV are at increased risk of developing cardiovascular disease (CVD), in part due to abnormal fat deposition and in part due to the actions of antiretroviral drugs themselves. Prevention of CVD in HIV-positive individuals is considered to be the most important medical intervention for long-term well-being, after highly active antiretroviral therapy (HAART) of course. Until recently, statins have been the cornerstone of medical management in this population.
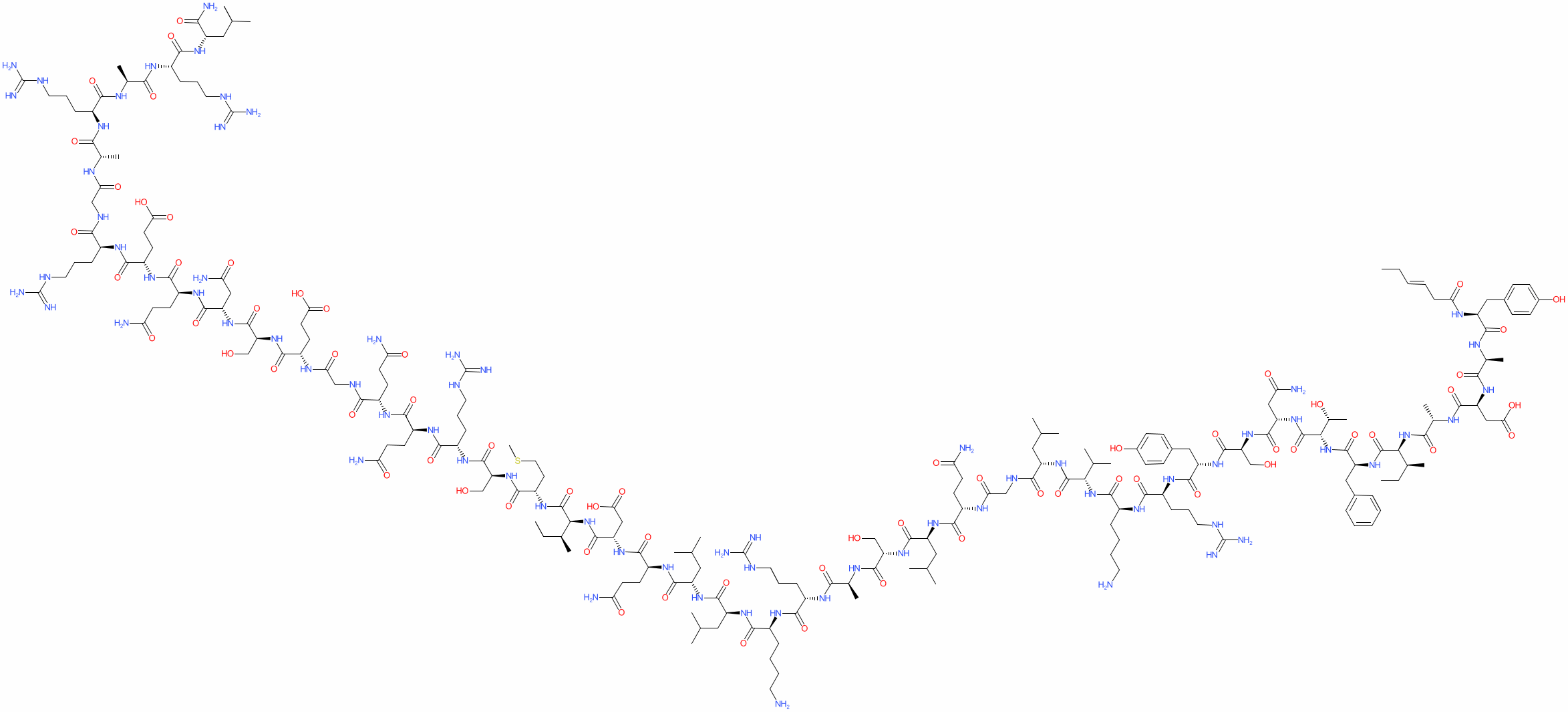
Research shows that tesamorelin, in addition to decreasing lipodystrophy, also reduces triglyceride levels, total cholesterol levels, and non-HDL-C levels in HIV-positive patients. A 15% reduction in visceral adipose tissue by tesamorelin correlates with a 50 mg decrease in trigylceride levels[3], [4].
It is worth noting that ectopic fat deposition, as seen in lipodystrophy, is associated with inflammation. Inflammation of any kind is a risk factor for CVD. Visceral adipose tissue, liver fat, and epicardial fat are all independently associated with increased risk of CVD. By reducing ectopic fat deposition, tesamorelin directly decreases inflammation and an individual’s risk for CVD.
Recent evidence suggests that HAART is associated with a number of endocrine and metabolic problems, including growth hormone (GH) deficiency. It appears that the pituitary gland is altered in HIV infection and, as a consequence, approximately one third of patients with HIV who are taking HAART have GH deficiency[5]. This may, to some extent, explain why lipodystrophy is so common in individuals with HIV and also why tesamorelin is such an effective treatment. Tesamorelin is a safer and more effective way to raise GH levels than administration of exogenous GH, particularly in HIV-positive individuals.
Peripheral nerve damage can be a consequence of injury, diabetes, or even surgical interventions. It often results in debilitating problems with both motor and sensory function in the affected area, but there is little that can be done to correct the problem because nerve cells are notoriously difficult to regenerate. Research, however, suggests that therapies based on growth hormone manipulation may improve peripheral nerve injury and increase both rate and extent of healing[6]. Tesamorelin is currently the leading candidate for such intervention, in part because it already has FDA approval.
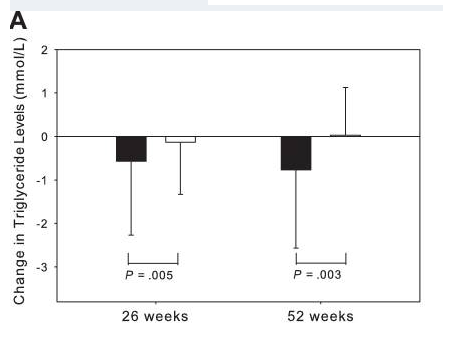
There is now evidence to suggest that GHRH analogues, like tesamorelin, are effective in enhancing cognition in patients suffering from the early stages of dementia. A large, randomized, double-blind, placebo-controlled study at the University of Washington School of Medicine, carried out over twenty weeks, suggests that tesamorelin and other GHRH analogues may impact dementia by increase gamma-aminobutyric acid (GABA) levels in the brain and by decreasing myo-insoitol (MI) levels[7]. These findings open up a pathway for using tesamorelin in the treatment of dementia, but also suggest new areas for scientists to explore as they look for a cure or a preventative.
Because it is FDA approved for use in humans, tesamorelin is an attractive peptide for ongoing clinical research. It is currently under review for its ability to reduce cardiovascular disease in HIV, improve healing of peripheral nerves following injury, and slow the progression of dementia. Clinical trials are already underway in several different areas.
Tesamorelin exhibits minimal side effects, low oral and excellent subcutaneous bioavailability in mice. Per kg dosage in mice does not scale to humans. Tesamorelin for sale at
The above literature was researched, edited and organized by Dr. Logan, M.D. Dr. Logan holds a doctorate degree from Case Western Reserve University School of Medicine and a B.S. in molecular biology.
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATONAL AND EDUCATIONAL PURPOSES ONLY.
The products offered on this website are furnished for in-vitro studies only. In-vitro studies (Latin: in glass) are performed outside of the body. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat or cure any medical condition, ailment or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.
PeptideGurus is a leading supplier of American-made research peptides, offering top-quality products at competitive prices. With a focus on excellence and customer service, they ensure a secure and convenient ordering process with global shipping.
CONTACT