



Categories: IGF-1 Proteins
What Is PT-141?
PT-141, also called bremelanotide, is sometimes referred to as the female Viagra because the peptide
was previously investigated in phase IIb human clinical trials for use in treating female hypoactive
sexual desire disorder (HSDD). PT-141 is a melanocortin that binds primarily to melanocortin 4
receptor (MC-4R) and MC-1R. In 2009, PT-141 was also investigated as a treatment for acute
hemorrhage. PT-141 is a derivative of another synthetic melanocortin, melanotan 2 (MT-2).
PT-141 Molecular Structure
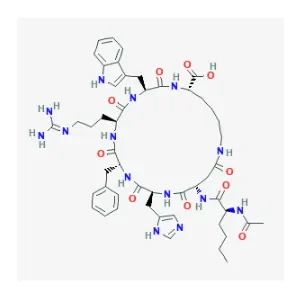
Sequence: Ac-Nle-Asp(1)-His-D-Phe-Arg-Trp-Lys(1)
Molecular Formula: C H N O
Molecular Weight: 1025.182 g/mol
PubChem CID: 9941379
CAS Number: 189691-06-3
PT-141 Research
PT-141 and Sexual Arousal
PT-141 is a unique peptide in that it stimulates the MC-4R, which is known to produce sexual arousal
in the central nervous system and influence sexual behavior . Studies in mice have shown that
agonist binding to MC-4R causes sexual arousal and increased copulation in both males and females
. Because PT-141 works via a different mechanism than drugs like Viagra, it is possible to treat
sexual arousal disorders in both men and women that stem from causes other than reduced blood
flow to the genitals.
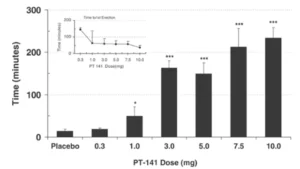
A study of men with erectile dysfunction (ED) who did not respond to sildenafil (Viagra) found that
roughly one third experienced adequate erection for sexual intercourse with PT-141 (administered via
nasal spray). There was also a strong dose-dependent response in the trial, indicating that PT-141 is
indeed effective in certain cases . This suggests that PT-141 could offer insight into correcting ED in
settings where sildenafil has failed and may offer insight into central causes of hypoactive sexual
desire.
Interestingly, PT-141 was pulled from clinical trials before it reached approval for use in women
suffering from HSDD. This is despite signs that the drug increased the number of satisfying sexual
events per month and decreased female sexual distress scores in a statistically significant manner
without any substantial side effects . Many experts who treat female sexual dysfunction (FSD) were
dismayed to find the peptide was not being advanced despite positive results. They point to a lack of
established endpoints for trials of FSD and socio-cultural biases against women’s sexual health as the
primary roadblocks that are inhibiting approval of what they see as much-needed therapies . They
hope that greater attention will be given to the topic and that the FDA will establish more concrete
guidelines for evaluating therapies like PT-141 that can offer benefit. These experts also expressed
dismay that the pharmacological treatments were not tested in conjunction with other established
means of treating sexual dysfunction as they believe that the combination may prove synergistic and
that peptides like PT-141 may be useful for overcoming initial barriers and jump-starting psychological
treatment modalities.
In 2017, partly in response to the outcry against the cessation of earlier trials, Phase II Reconnect
trials were launched using subcutaneous injections of PT-141 for FSD. The newest version of PT-141,
called Rekynda, may soon be available for use in the United States. It would be legal to use PT-141
off-label, at that point, to treat both male and female sexual dysfunction . These new trials have relied
on the kind of modified endpoints that experts in FSD have touted as beneficial to seeing these kinds
of treatments approved.
PT-141 and Hemorrhage
In 2009, PT-141 was modified slightly and investigated as a potential treatment for hemorrhagic shock.
Because PT-141 binds to both MC-1R and MC-4R, it reduces ischemia and protects tissues against
inadequate blood supply in the setting of hypovolemic (hemorrhagic) shock. The drug, when
administered intravenously, does not produce substantial side effects. It was last in phase IIb trials.
The modified version of PT-141 is referred to as PL-6983.
PT-141 and Infection
The MC-1R has been found, in a rat model of a specific fungal infection, to possess important anti fungal and anti-inflammatory properties . This is of particular importance because current anti fungals are limited in terms of their mechanism of action and all produce serious and treatment-limiting side effects in certain patients. Having an alternative to use in the treatment of fungal infections could
reduce morbidity and mortality substantially, especially in patients with immune compromise.
PT-141 and cancer
The MC-1R receptor is an important stimulus of DNA repair pathways and thus is ofinterest in cancer treatment and prevention[1o]. Research shows that people with variantsof MC-1R are at increased risk for both basal cell and squamous cell carcinoma 1.Altered PT-141 may be able to correct the problems experienced as a result of thesevariants and prevent or treat these cancers.
Research Directions
Right now, PT-141 has received widespread and intense attention as a treatment forsexual dysfunction. There is, however, a great deal of potential research outside of sexualdysfunction and hemorrhage that PT-141 could be applied to. For instance, MC-4R iswell-known to be defective or missing in certain cases of obesity and may account for asmuch as 6% of all cases of early-onset obesity. PT-141 offers a unique means ofexploring this particular cause of obesity and potentially illustrating a pathway forintervention. MC-1R plays roles in both pain and inflammation as well as kidneypathology and the spread of infection. There is a plethora of available research that PT.141 could help to shed light on.
PT-141 exhibits minimal side effects, low oral and excellent subcutaneous bioavailabilityin mice. Per kg dosage in mice does not scale to humans. PT-141 for sale at PeptideSciences is limited to educational and scientific research only, not for humanconsumption. Only buy PT-141 if you are a licensed researcher.
Article Author
The above literature was researched, edited and organized by Dr. Logan, M.D. Dr. Loganholds a doctorate degree from Case Western Reserve University School of Medicine anda B.S.in molecular biology.
Scientific Journal Author
Dr. Sheryl A. Kingsberg is the chief of behavioral medicine at University Hospitals CaseMedical Center and professor in Reproductive Biology and Psychiatry at Case WesternReserve University. Her areas of clinical specialization include sexual medicine, femalesexual disorders, cognitive behavioral psychotherapy, menopause, pregnancy andpostpartum mood disorders, psychological aspects of infertility, and psychological andsexual aspects of cancer. Dr. Kingsberg’s primary research interests are in treatments forfemale sexual disorders and the psychological aspects of infertility and menopause. Sheled a randomized, placebo-controlled dose-finding trial for PT-141. She has numerouspublications in many national and international journals, sits on the editorial board ofMenopause and has authored numerous chapters on topics including perimenopauseand sexuality, oocyte donation, infertility and aging, the treatment of psychogenic erectiledysfunction and sexuality after cancer. Dr. Kingsberg received her PhD from theUniversity of South Florida in Tampa and completed her fellowship in sexual medicine atUniversity Hospitals Case Medical Center. She is an active member in a number ofnational and international organizations including the American Psychological Associationand the American Society for Reproductive Medicine. She currently sits on the Board ofTrustees of The North American Menopause Society, and serves as the current treasurerof the Society for Assisted Reproductive Technologies. Dr. Kingsberg s a past president ofThe International Society for the Study of Women’s Sexual Health.
Dr. Sheryl A. Kingsberg is being referenced as one of the leading scientists involved inthe research and development of PT-141. In no way is this doctor/scientist endorsing oradvocating the purchase, sale, or use of this product for any reason. There is no affiliationor relationship, implied or otherwise, between PEPTIDE GURUS and this doctor. Thepurpose of citing the doctor is to acknowledge, recognize, and credit the exhaustiveresearch and development efforts conducted by the scientists studying this peptide. Dr.Kingsberg is listed in [12] under the referenced citations.
Referenced Citations
1.M. Sandrock, A. Schulz, C.Merkwitz,T. Schöneberg, K. Spanel-Borowski, and A.Ricken, “Reduction in corpora lutea number in obese melanocortin-4-receptor-deficient mice,”Reprod. Biol. Endocrinol.RBE, vol.7, p.24, Mar. 2009.
2.R.C.Rosen, L.E.Diamond, D.C. Earle, A. M. Shadiack, and P. B. Molinoff.*Evaluation of the safety, pharmacokinetics and pharmacodynamic effects ofsubcutaneously administered PT-141, a melanocortin receptor agonist, in healthymale subjects and in patients with an inadequate response to Viagra,” Int. J. lmpotRes.,vol.16,no.2,pp.135-142,Apr.2004.[PubMed]
3.H.Wessells, V. J. Hruby, J. Hackett, G. Han, P. Balse-Srinivasan, and T. W.Vanderah,“Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2 induces penile erection viabrain and spinal melanocortin receptors,” Neuroscience, vol. 118, no. 3, pp. 755-762.2003.[PubMed]
4.A.-S. Rössler, J. G. Pfaus, H. K. Kia, J. Bernabé, L.Alexandre, and F. Giuliano,“The melanocortin agonist, melanotan ll, enhances proceptive sexual behaviors inthe female rat,”Pharmacol. Biochem.Behav., vol.85, no.3, pp.514-521, NOV.2006.[PubMed]
5.M.R.Safarinejad and S.Y. Hosseini, “Salvage of sildenafil failures withbremelanotide: a randomized, double-blind, placebo controlled study,” J. Urol., vol.179,no.3,pp.1066-1071,Mar.2008.[PubMed]
6.A. H. Clayton et al., “Bremelanotide for female sexual dysfunctions inpremenopausal women: a randomized, placebo-controlled dose-finding trial,Womens Health Lond.Engl., vol.12,no.3, pp.325-337,2016.[PubMed]
7.M.K. Miller, J.R. Smith, J. J. Norman, and A. H. Clayton,“Expert opinion onexisting and developing drugs to treat female sexual dysfunction,” Expert Opin.Emerg.Drugs, vol.23,no.3,pp.223-230,2018.[PubMed]
8.“AMAG Pharmaceuticals and Palatin Technologies Enter Into Exclusive LicensingAgreement for North American Rights to RekyndaTM (bremelanotide), a PotentialTreatment for a Common Female Sexual Disorder -AMAG Pharmaceuticals.[MarketWatchl]
9.H. Ji et al.. “The Synthetic Melanocortin (CKPV)2 Exerts Anti-Fungal and Anti.Inflammatory Effects against Candida albicans Vaginitis via Inducing MacrophageM2 Polarization,”PLoS ONE, vol.8, no.2,Feb.2013.[PLOS ONE]
10.V. Maresca, E. Flori, and M. Picardo, “Skin phototype: a new perspective,” PigmentCell Melanoma Res., vol.28,no.4,pp.378-389,Jul.2015.[PubMed]
11.L. Feller, R. a. G. Khammissa, B. Kramer, M. Altini, and J. Lemmer, “Basal cellcarcinoma, squamous cell carcinoma and melanoma of the head and face,” HeadFace Med., vol.12,p.11,Feb.2016.[PubMed]
12.Clayton AH, Althof SE, Kingsberg S, et al. Bremelanotide for female sexualdysfunctions in premenopausal women: a randomized, placebo-controlled dose.finding trial. Womens Health (Lond), 2016:12(3):325-337. doi:10.2217/whe-2016-0018
13.T.R. McMillan, M. A. M. Forster, L.l. Short, A. P. Rudecki, D.L. Cline, and S. L.Gray, “Melanotan ll, a melanocortin agonist, partially rescues the impairedthermogenic capacity of pituitary adenylate cyclase-activating polypeptide deficientmice,Exp.Physiol.,vol.106,no.2,pp.427-437,Feb.2021, doi:10.1113/EP088838.”
14.C.Spana, R. Jordan, and S. Fischkoff, “Effect of bremelanotide on body weight ofobese women: Data from two phase 1 randomized controlled trials,Diabetes ObesMetab., vol.24,no.6,pp.1084-1093, Jun.2022, doi: 10.1111/dom.14672
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE AREFOR INFORMATONAL AND EDUCATIONAL PURPOSES ONLY.
The products offered on this website are furnished for in-vitro studies only. In-vitro studies(Latin: in glass) are performed outside of the body. These products are not medicines ordrugs and have not been approved by the FDA to prevent, treat or cure any medicalcondition, ailment or disease. Bodily introduction of any kind into humans or animals isstrictly forbidden by law.
PeptideGurus is a leading supplier of American-made research peptides, offering top-quality products at competitive prices. With a focus on excellence and customer service, they ensure a secure and convenient ordering process with global shipping.
CONTACT