Бесплатно (1) 30 мл Бактериостатическая вода
с квалифицированными заказами более $500 доллар США.
(исключает капсульные продукты, косметические пептиды, промокоды и доставка)
ТБ-500 (Тимозин Бета-4) это 43 аминокислотная пептидная последовательность. В моделях животных, Было показано, что тимозин бета-4 улучшает рост кровеносных сосудов., регулировать заживление ран, уменьшить воспаление, и уменьшают окислительное повреждение сердца и центральной нервной системы.. Тимозин-бета-4 играет защитную роль., восстановление тканей, регенерация и ремоделирование травмированных или поврежденных тканей. Он также представляет активный интерес к исследованиям в области борьбы со старением..
Использование продукта: НАСТОЯЩИЙ ПРОДУКТ ПРЕДНАЗНАЧЕН ТОЛЬКО В качестве ИССЛЕДОВАТЕЛЬСКОГО ХИМИКАТА.. Это обозначение позволяет использовать исследовательские химикаты исключительно для испытаний in vitro и лабораторных экспериментов.. Вся информация о продуктах, доступная на этом сайте, предназначена только для образовательных целей.. Телесное введение любого вида в организм человека или животного строго запрещено законом.. С этим продуктом должны обращаться только лицензированные, квалифицированные специалисты. Этот продукт не является лекарством, еда, или косметическое средство и не может иметь неверную маркировку, неправильно использовался или ошибочно маркировался как наркотик, еда или косметика.
ТБ-500 (Тимозин Бета-4)
Thymosin Beta 4, or TB-500, is a synthetic version of a naturally occurring 43-amino acid peptide present in nearly all human and animal cells studied.
А 2010 study in the Annals of the New York Academy of Sciences supported TB500’s potential for cardiac muscle repair following injury, eg, after myocardial infarction (острое сердечно-сосудистое заболевание). Recognizing the limitations of stem cell therapy for this application, TB500 was found to inhibit myocardial cell death, stimulate blood vessel growth, and activate cardiac processes that encouraged the heart to heal following injury. The investigation showed that TB500 may be the first agent which can actively recover injured cardiac muscle following heart attack. This is further supported with prior mouse studies in 2004 showing cardiomyocyte migration, survival and repair of myocardial damage. [1] [2]
Filamentous actin (F-actin, or actin) forms polymers that thicken sputum, adversely affecting cystic fibrosis patients. TB500 was studied in a population of CF patients, showing a dose- and time-dependent decrease in cohesivity of sputum after administration of TB500 when combined with dornase alfa. The combination therapy showed a 71% improvement in mucociliary transport of mucus, and a 44% improvement in cough transport of mucus. [3]
TB500 is known to stimulate myoblasts and myocytes (muscle generating cells). Mitochondrial RNA levels of TB500 have been shown to increase following muscle injury, helping to regenerate muscle fibers and address inflammation in the injured location. The data support muscle injury causing increased local production of TB500, promoting migration of incoming myoblasts to accelerate skeletal muscle regeneration. [4]
ТБ-500 (Тимозин Бета-4) 2мг
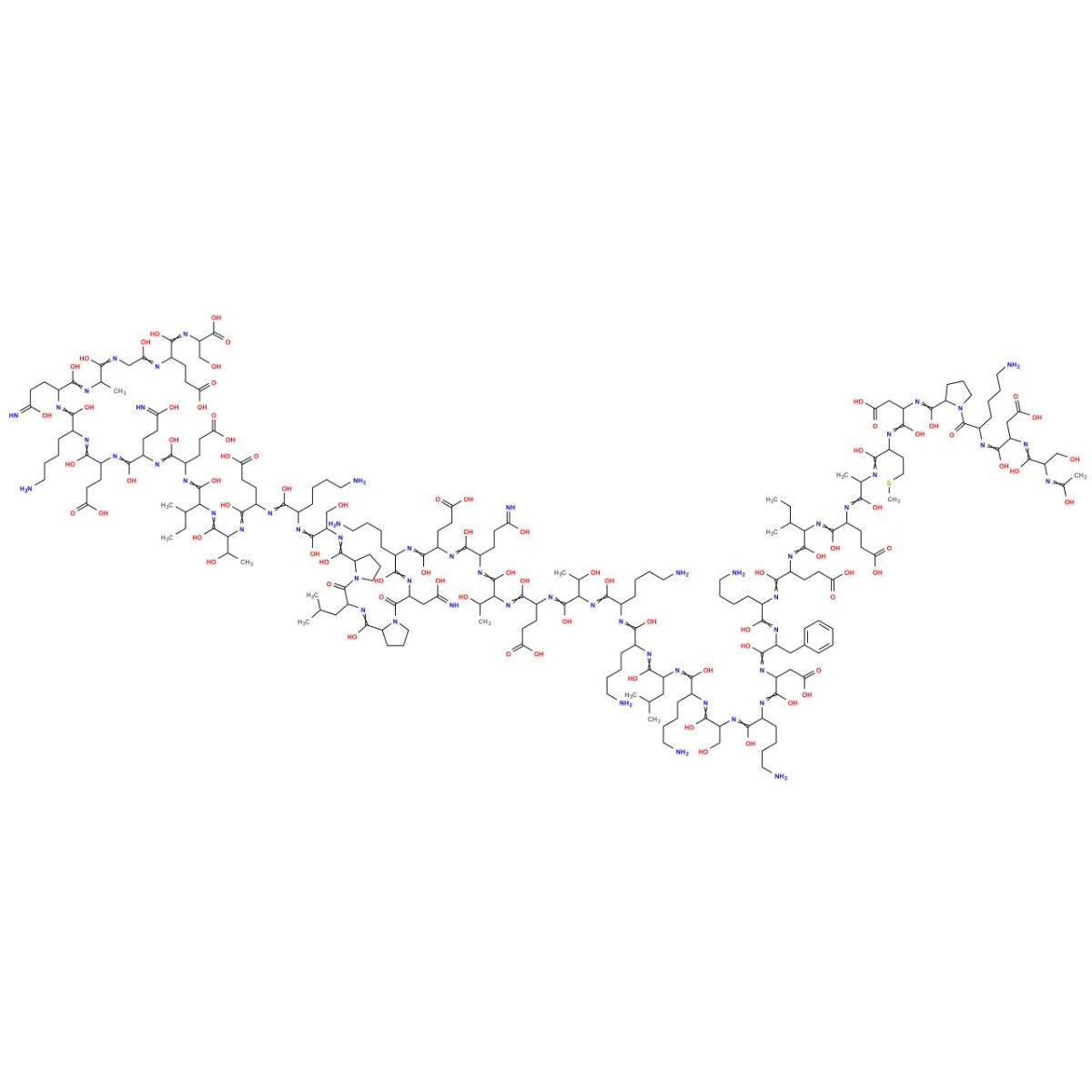
Последовательность: Ac-Ser-Asp-Lys-Pro-Asp-Met-Ala-Glu-Ile-Glu-Lys-Phe-Asp-Lys-Ser-Lys-Leu-Lys-Lys-Thr-Glu-Thr-Gln-Glu-Lys-Asn-Pro-Leu-Pro-Ser-Lys-Glu-Thr-Ile-Glu-Gln-Glu-Lys-Gln-Ala-Gly-Glu-Ser
Молекулярная формула: C212H350N56O78S
Молярная масса: 4963.4408
CAS number: 77591-33-4
PubChem: CID 16132341
Синонимы: Thymosin Beta 4
TB-500 Research Studies
A study completed in 2009 using .01% TB500 w/w eye drops demonstrated increased speed of healing following eye surgery in diabetics. This population was specifically investigated due to well known healing complications in diabetics, and their predilection for eye issues, specifically diabetic retinopathy. The study showed no serious side effects in any patient, although headache, dizziness and insomnia occurred more in the study group than the control. Of specific interest, human diabetic retinopathic corneas express substantially less endogenous TB500 than a normal cornea, suggesting an inherent deficiency in ability for diabetics to heal following eye injury or surgery. [5] Сходным образом, chronic dry-eye patients were treated with TB500 eye drops, and all in the study had improvement in symptoms, with rare and minimal complications. [6]
TB500 was studied in the setting of chronic hepatitis B combined with nonalcoholic fatty liver disease (NAFLD). While TB500 had no correlation with HepB Virus levels nor liver function tests (AST, ALT, TG), there was a negative correlation with inflammation and fibrosis scores – meaning that the lower the level of TB500, the worse the inflammation and fibrosis. This provided preliminary data that TB500 may be beneficial in the setting of certain liver diseases. [7]
A human voluntary safety and tolerance study was performed in 2010, and showed that intravenous (IV) doses from 42mg up to 1260mg daily for 14 days had no treatment related adverse effects, and no evidence of dose related toxicity. [8]
Rhinovirus (a common cold virus) was introduced to healthy volunteers, and blood tests were taken during a five-day period. This showed that serum cortisol rose along with thymosin alpha 1 and TB500 on the fifth day following intentional exposure. Simultaneously, t-lymphocytes (CD3+), cytotoxic/suppressor (CD8+), and natural killer (CD16+) cells all rose. This suggests a cooperative effect among systemic and cellular immune response related to thymosin and respiratory virus exposure. [9]
Kidney disease often includes inflammation, фиброз, and complications from diabetes. TB500 has been studied in mice with and without kidney disease; in healthy mice, levels of TB500 did not affect healthy kidney tissue, but in those with known kidney disease, low levels of TB500 correlated with worsening disease. That is, low levels of natural TB500 accelerated kidney disease. The study suggested that endogenous TB500 levels function to protect the kidney and slow disease progression. [10]
TB500 has been studied specifically in healing of skin and wounds, showing acceleration in healing of skin in burns, diabetic ulcers, elderly subjects, pressure ulcers, stasis ulcers, and epidermolysis wounds, in both animal and human subjects. The study shows improvement in angiogenesis (blood vessel formation), anti-inflammatory activity, and to increase platelet aggregation at wound sites. [11]
A meta-analysis (study of studies) conducted in 2015 showed broad applicability of TB500 in various disease processes, including improvement of tissue regeneration, repair of the heart after heart attack, healing of the brain following stroke, trauma and neurological diseases. Further it showed clinical improvement in kidney and liver diseases, and repair of spinal cord, bone and ligament injuries, as well as reducing consequences of aging and viral infection. [12]
TB500 treated mice were found to have substantially improved strength in healed fractures compared to untreated mice. Treated mice showed a 41% increase in peak force to failure, and healed fractures were 25% stiffer than untreated mice. 21-day post-fracture imaging showed improvement up to 26% better than untreated subjects. They also showed 23% smaller callus, and a 31% increase in trabecular bone area/total callus area. Overall TB500 demonstrated substantially faster healing, with better-healed injury sites when compared to placebo subjects. [13]
Spinal cord injury has been studied in rats, with potential applicability to human subjects. В 2014 изучать, TB500 was administered to rats 30 минуты, 3 дни, или 5 days after mild compression induced spinal cord injury in rats. Locomotor and behavioral assessments were all markedly improved with the TB500 treated group. Inflammatory cytokines were reduced, the size of scars were markedly reduced, and levels of myelin proteins were 57.8% above the control group. The study suggested that, with the known safety of TB500, that it should be considered for treatment of spinal cord injury in humans. [14]
While no animal model perfectly mimics humans for head injury research, rats are considered a close second due to our understanding of their behaviors. TB500 has been studied in traumatic brain injury (ЧМТ), and has been demonstrated to markedly improve function after TBI. TB500 exerts both neuroprotective and neurorestorative effects on rats, including proliferation of blood flow, new brain cells, and new connections between brain cells. [15]
Multiple sclerosis has also been shown, in rat models, to benefit from administration of TB500. Zhang et al, in their 2016 изучать, showed that administration of TB500 significantly increased the number of newly generated oligodendrocytes and reduced axonal damage in the mice compared with control group. Важно, the newly generated oligodendrocytes remyelinated axons, and this correlated with functional improvement. [16]
Рекомендации
- Ann N Y Acad Sci. 2010 апрель;1194:87-96. Thymosin beta4 and cardiac repair. Shrivastava S1, Srivastava D, Olson EN, DiMaio JM, Bock-Marquette I.
- Природа. 2004 ноябрь 25;432(7016):466-72. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Bock-Marquette I1, Saxena A, White MD, Dimaio JM, Srivastava D.
- Chest. 2006 ноябрь;130(5):1433-40. Thymosin beta4 sequesters actin in cystic fibrosis sputum and decreases sputum cohesivity in vitro. Rubin BK1, Kater AP, Goldstein AL.
- J Biochem. 2011 Ян;149(1):43-8. дои: 10.1093/jb/mvq115. Электронная публикация 2010 Сентябрь 29. Muscle injury-induced thymosin β4 acts as a chemoattractant for myoblasts. Tokura Y1, Nakayama Y, Fukada S, Nara N, Yamamoto H, Matsuda R, Hara T.
- https://clinicaltrials.gov/show/NCT00598871
- Ann N Y Acad Sci. 2010 апрель;1194:199-206. дои: 10.1111/j.1749-6632.2010.05471.x. Treatment of chronic nonhealing neurotrophic corneal epithelial defects with thymosin beta4. Dunn SP1, Heidemann DG, Chow CY, Crockford D, Turjman N, Angel J, Allan CB, Sosne G.
- Medicine (Балтимор).2016 декабрь;95(52):e5763. дои: 10.1097/доктор медицинских наук.0000000000005763. The expression of thymosin β4 in chronic hepatitis B combined nonalcoholic fatty liver disease. Liang J1, Cai W, Han T, Jing L, Ma Z, Gao Y.
- Ann N Y Acad Sci. 2010 апрель;1194:223-9. дои: 10.1111/j.1749-6632.2010.05474.x. A randomized, placebo-controlled, single and multiple dose study of intravenous thymosin beta4 in healthy volunteers. Ruff D1, Crockford D, Girardi G, Zhang Y.
- Lymphokine Res. 1989 Winter;8(4):383-91. Modulation of thymosin alpha 1 and thymosin beta 4 levels and peripheral blood mononuclear cell subsets during experimental rhinovirus colds. Hsia J1, Sztein MB, Naylor PH, Simon GL, Goldstein AL, Hayden FG.
- Kidney Int. 2016 ноябрь;90(5):1056-1070. дои: 10.1016/j.kint.2016.06.032. Электронная публикация 2016 август 26. Loss of endogenous thymosin β4 accelerates glomerular disease. Vasilopoulou E1, Kolatsi-Joannou M1, Lindenmeyer MT2, White KE3, Robson MG4, Cohen CD2, Sebire NJ1, Riley PR5, Winyard PJ1, Long DA6.
- Vitam Horm. 2016;102:251-75. дои: 10.1016/bs.vh.2016.04.005. Электронная публикация 2016 Может 24. Thymosin β4 Promotes Dermal Healing. Kleinman HK1, Sosne G2.
- Expert Opin Biol Ther. 2015;15 Дополнение 1:S139-45.doi:10.1517/14712598.2015. 1011617. Электронная публикация 2015 июнь 22. Advances in the basic and clinical applications of thymosin β4. Goldstein AL1, Kleinman HK.
- J Orthop Res. 2014 октябрь;32(10):1277-82. дои: 10.1002/jor.22686. Электронная публикация 2014 июль 8. Thymosin β4 administration enhances fracture healing in mice. Brady RD1, Grills BL, Schuijers JA, Ward AR, Tonkin BA, Walsh NC, McDonald SJ.
- Нейрофармакология. 2014 октябрь;85:408-16. дои: 10.1016/j.neuropharm.2014.06.004. Электронная публикация 2014 июнь 14. Beneficial effects of thymosin β4 on spinal cord injury in the rat. Cheng P1, Kuang F1, Zhang H1, Ju G2, Wang J3.
- Ann N Y Acad Sci. 2012 октябрь;1270:51-8. дои: 10.1111/j.1749-6632.2012.06683.x. Neuroprotective and neurorestorative effects of thymosin β4 treatment following experimental traumatic brain injury. Xiong Y1, Mahmood A, Meng Y, Zhang Y, Zhang ZG, Morris DC, Chopp M.
- Neurobiol Dis. 2016 апрель;88:85-95. дои: 10.1016/j.nbd.2016.01.010. Электронная публикация 2016 Ян 12. Thymosin beta4 promotes oligodendrogenesis in the demyelinating central nervous system. Zhang J1, Zhang ZG2, Li Y2, Lu M3, Zhang Y2, Elias SB2, Chopp M4.
ВСЕ СТАТЬИ И ИНФОРМАЦИЯ О ПРОДУКЦИИ, ПРЕДОСТАВЛЕННЫЕ НА ЭТОМ ВЕБ-САЙТЕ, ПРЕДНАЗНАЧЕНЫ ТОЛЬКО ДЛЯ ИНФОРМАЦИОННЫХ И ОБРАЗОВАТЕЛЬНЫХ ЦЕЛЕЙ..
Продукты, предлагаемые на этом сайте, предназначены только для исследований in vitro.. Исследования in vitro (латинский: в стекле) выполняются вне тела. Эти продукты не являются лекарствами или лекарствами и не были одобрены FDA для предотвращения, лечить или вылечить любое заболевание, недуг или болезнь. Телесное введение любого вида в организм человека или животного строго запрещено законом..